Adding Hcl to Mg Chemical or Physical
Cl 2 H 2 2 HCl. No new kind of matter is formed in a physical change.

Nittygrittysci Posted To Instagram Chemical Bonds And Equations Include Interactive Science Notebook Physical Science Interactive Notebook Teaching Chemistry
Why is reaction of HCl and Zn chemical change.

. Add 40 ml of distilled water and label the conical flask with the concentration of the HCL poured. Burning is a chemical change because a new substance is formed. Hydrogen chloride is produced by combining chlorine and hydrogen.
What if 00800 g of Mg had been used. Physical state and appearance. Part B Salt and Sand done as a class together Mix a spatula full of sodium chloride and sand on a clean paper towel.
Heat the test tube gently in a burner flame and watch carefully for changes. However HCl aq found in the stomach has a pH level of 1 to 2. But it is not always easy to look at a change and decide if it is a physical or chemical one.
Concentrated HCl aq has a pH level of 0. Place 5 mL of dilute hydrochloric acid HCl in a large test tube. This type of reaction can be written as.
2HCl aq Mg s MgCl2 aq H2 g. This is as shown in the equation below. Is adding HCl to Mg chemical or physical.
Up to 24 cash back Place an unburned 1-cm strip of Mg and the burned combustion product ash into two separate test tubes. Wait until all the Mg has reacted add more acid if necessary and then pour down the sink with running water. Ripping the Magnesium is a physical change because we are changing the size and shape but not changing what the substance is.
Add 10 drops of 6M hydrochloric acid to each tube. Cut a small piece of Mg Magnesium ribbon. A single replacement reaction involves one element replacing another element in a compound.
When heating a test tube never point the mouth of it at yourself or anyone else. Consult your text if necessary. The initial substance reactants disappear.
Inhaling this compound causes severe coughing. The average removal efficiency of HCl on MgFe HTOs is affected primarily by temperature. Teaching notes The equation for the reaction is.
This is a single replacement. Here are some indicators of a chemical change. Carefully feel the bottom of each test tube.
Flask 3 00500 mol Mg 01 mol HCl stoichiometric HClMg ratio Flask 4 01000 mol Mg 01 mol HCl excess Mg. Mg OH2 s 2 HCl MgCl2 aq 2 H2Ol MgCl 2 as a precursor. Produced if 00400 g of Mg had been reacted with 500 mL of 10 M HCl.
Place the piece of Mg in a well in the well plate and add a few drops of HCl Hydro chloric Acid. A BC AC B Aim To see if the concentration of hydrochloric acid will. This reaction can give a very pure product eg.
Test conditions included 1 reconstituted gemcitabine at a concentration of 38 mgmL as the. For use in the food industry. Stir the solution using a glass stir rod.
Flask 1 and 2 are limited by smaller quantities of Mg. For stance magnesium metal reacts with hydrochloric to form magnesium chloride salt while displacing hydrogen from the acid as hydrogen gas. Up to 24 cash back Here are some of the general physical properties of HCl aqueous.
Feel the bottom of each test tube. The adsorption of HCl on MgFe HTOs was studied from 350 to 650 C. What is the color of the solution now.
Place a thermometer in the acid and then add 5 mL of. As the reaction is exothermic the installation is called an HCl oven or HCl burner. Magnesium metal in form of a ribbon or powder reacts with acids rapidly than water liberating hydrogen gas.
Flask 3 will react to use both reagents evenly and completely. Write a balanced chemical equation including phase labels of the reaction that took place in this experiment. Put half of your sucrose sample into a test tube.
HCl aq Mg s MgCl2 aq H2 g This is a single replacement reaction. The balanced formula for this is. Add a drop of 6 M hydrochloric acid solution to the test tube and stir the solution.
Take the temperature of the solution before shaking and after shaking. The elements Magnesium and Oxygen bond to form a white powder- Magnesium Oxide. 0120 g of Mg.
Yes because acids react with active metals by giving off hydrogen gas. The reaction is as follows. Add 5 drops of 6M HCl hydrochloric acid to each tube.
10858 C 760 mm Hg for 2022 HCl in water 83 C 760 mm. Measure 40 ml of 3M HCl using a clean dry measuring cylinder and pour into a clean 100 ml conical flask. A chemical change has occurred when a substance changes into a new substance.
Add 2 mL of salt KCl solution to 2 mL of silver nitrate solution AgNO3. To evaluate the physical and chemical stability of gemcitabine hydrochloride Gemzar-Eli Lilly and Company solutions in a variety of solution concentrations packaging and storage conditions. Calcined MgFe hydrotalcite-like compounds HTLs hydrotalcite-like oxides HTOs have shown potential performance for removing hydrochloric acid HCl from flue gas at medium-high temperatures.
Problems faced in locomotive boilers. Liquid colourless- light yellow Odor. The chemical equation between magnesium ribbon and hydrochloric acid can be written as.
Continue timing until no more gas appears to be given off. Use extreme care when handling acids. MgCl2 Mg Cl2 Health hazards Chloromagnesite is non-combustible but when heated liberates irritating toxic gas or fumes.
Continue adding hydrochloric acid solution to the test tube one drop at a time recording the color of the solution after each drop of hydrochloric acid solution is added. Burning is adding Oxygen gas. The resulting hydrogen chloride gas is absorbed in deionized water resulting in chemically pure hydrochloric acid.
When HCl and Zn react they form two. Magnesium hydrochloric acid magnesium chloride hydrogen Mg s 2HCl aq MgCl 2 aq H 2 g Students follow the rate of reaction between magnesium and the acid by measuring the amount of gas produced at 10 second intervals. Mgs 2HCLaq MgCl2aq H2g Magnesium hydrochloric acid Magnesium Chloride Hydrogen Magnesium will react with hydrochloric acid because it is higher in the reactivity series than hydrogen.
Add 05 g of sodium acetate NaC2H3O2 to 5 mL of distilled water in a test tube. Repeat step 5 and 6 for 2M 15M 1M and 05M HCL and keep all the acids ready on the working bench.
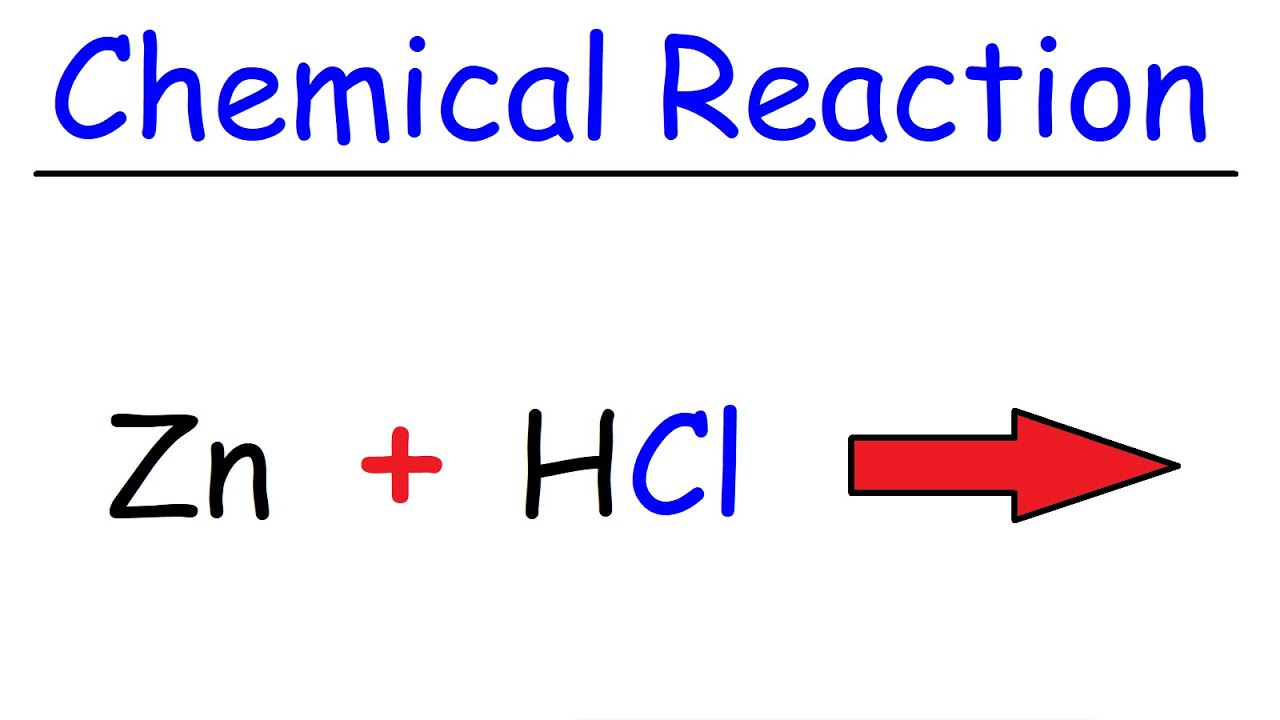
Zn Hcl Reaction Zinc Hydrochloric Acid Youtube

What Is The Reaction Between Magnesium And Hydrochloric Acid Quora

Magnesium And Hydrochloric Acid Lab Youtube
Laduviglusib Chir 99021 Hcl 99 Hplc Selleck Gsk 3 Inhibitor
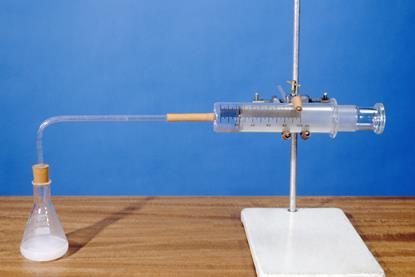
The Rate Of Reaction Of Magnesium With Hydrochloric Acid Experiment Rsc Education

Magnesium And Hydrochloric Acid Chemdemos
Hydrogen Chloride Vs Hydrochloric Acid Video Lesson Transcript Study Com

Chart Acids And Bases Chemistry Lessons Chemistry Basics Teaching Chemistry

Balancing Chemical Equations Practice Stations Chemical Equation Physical Science High School Equations

Net Ionic Equation For Mg Hcl Magnesium Hydrochloric Acid Youtube

Type Of Reaction For Hcl Mg Oh 2 Mgcl2 H2o Youtube
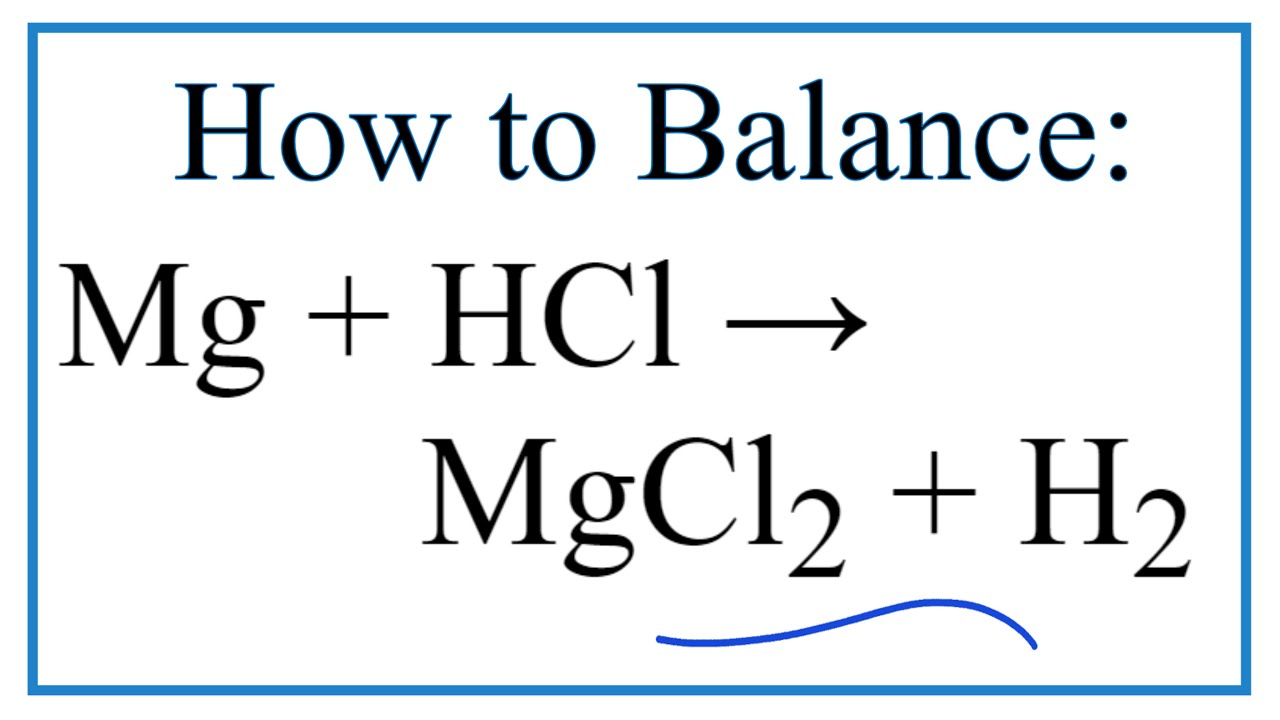
How To Balance Mg Hcl Mgcl2 H2 Magnesium Hydrochloric Acid Youtube

How To Balance Mg Oh 2 Hcl Mgcl2 H2o Magnesium Hydroxide Hydrochloric Acid Youtube

Hydrochloric Acid And Magnesium What Do You See When You React Hcl And Mg Youtube

How To Balance Caco3 Hcl Cacl2 Co2 H2o Chemistry Balance Molecules

Type Of Reaction For Mg Hcl Mgcl2 H2 Youtube

How To Balance Mgo Hcl Mgcl2 H2o Magnesium Oxide Hydrochloric Acid Youtube

The Rate Of Reaction Of Magnesium With Hydrochloric Acid Experiment Rsc Education

Is H2o Polar Or Nonpolar Water Polarity Of Water Polar Molecules
Comments
Post a Comment