Which Best Describes Gas Under Greater Than Atmospheric Pressure
Typically for respiration other pressure values are discussed in relation to atmospheric pressure. During exhalation the intrapulmonary pressure is greater than atmospheric pressure.

Factors Affecting Gas Pressure Read Chemistry Ck 12 Foundation
When the pleural cavity is damaged or ruptured and the intrapleural pressure becomes greater than the atmospheric pressure pneumothorax may ensue.
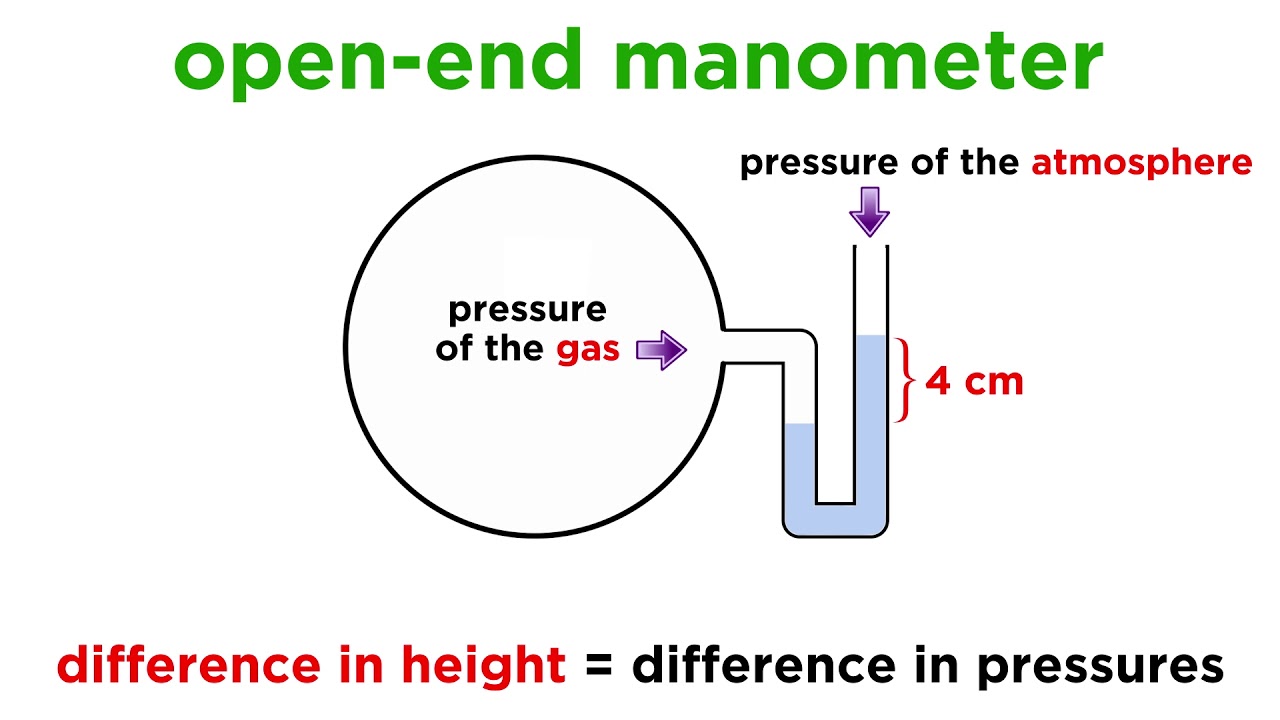
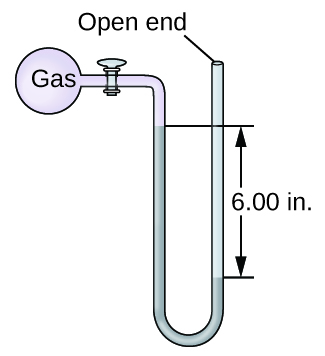
. Volume and moles gas. The pressure of the gas equals the hydrostatic pressure due to the pressure of the atmosphere at sea level minus a column of mercury of height 137 cm. The amount of gas molecules in the air decreasesthe air becomes less dense than air nearer to sea level.
As volume decrease pressure increase. A gauge pressure higher than ambient pressure is referred to as positive pressure. Intrapleural pressure is different from intrathoracic pressure.
According to the kinetic theory of gases. Expiration conversely occurs when the intrapulmonary pressure is greater than the atmospheric pressure. These tiny air sacs are the site where gas exchange between inspired air and the blood takes place.
Less than the pressure in the alveoli and less than atmospheric pressure C. What is the volume of a mole of an ideal gas under standard conditions. HBO is the use of increased oxygen concentrations under greater than normal atmospheric pressure.
Greater than the pressure in the alveoli and less than atmospheric pressure. The pressure of gas in your lungs is inversely proportional to the volume in your lungs. Which of these best.
Hyperbaric oxygenation Also known as hyperbaric oxygen therapy. One atm is equal to 760 mm Hg which is the atmospheric pressure at sea level. Repeat step 2 for the other sensor 2 and adjust if rquired.
Therefore negative pressure is pressure lower than the atmospheric pressure whereas positive pressure is pressure that it is greater than the atmospheric pressure. During inspiration the intrapleural pressure is higher than during expiration. If the volume of the gas decreased to 20 L calculate its new pressure.
The pressure on the left is due to the gas and the pressure on the right is due to the atmospheric pressure minus 137 cm Hg. Maybe your confusion stems from a belief that gases must be produced by boiling liquids since a liquid must have a vapor pressure equal to the external. Intrapulmonic pressure becomes higher than intrapleural pressure.
760 mm Hg 137 mm Hg 623 mm Hg. The substances are always liquid. At constant temperature of 35 ºC a sample of gas occupies a volume of 50 L and has a pressure of 2 atm.
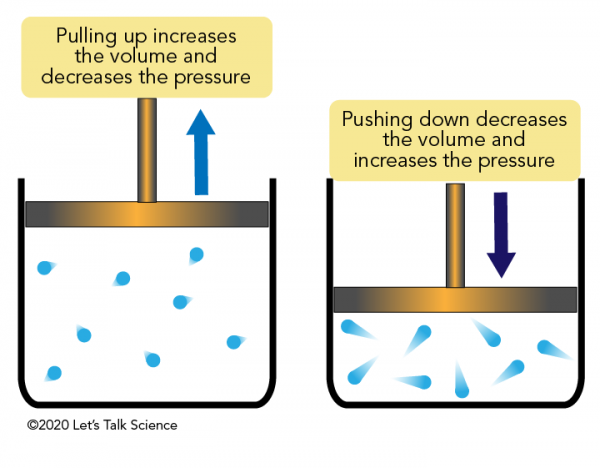
A measure of the force exerted by a gas above a liquid. Decreasing the volume of a gas increases the pressure of the gas. 28 Why do we not feel air pressure Although there is a tall column of air above US Class 8.
The average kinetic energy of water molecules is greater in. 26 Which statement best describes the relationship between wind and air pressure. Volume and pressure in gases the gas laws Boyles law.
This is what meteorologists and mountaineers mean by thin air. Which way does air flow when alveolar pressure is greater than atmospheric pressure. As alveolar volume increases alveolar pressure decreases.
Intrapulmonic pressure becomes less than atmospheric pressure. How to use equation 1 to calculate gas volume or pressure. Select the phrase that best describes PO2 at the arterial ends of the pulmonary capillaries.
The atmospheric pressure is greater at the top of the mountain than. Which of the following characteristics accurately describes Boyles law. If the measured pressure is below atmospheric pressure it is called negative or vacuum gauge pressureJun 18 2013.
For example at room temperature the pressure of water vapor over a pond or lake might be around 25 torr. February 8 2022 What best describes an unsubsidized federal loan QA February 8 2022. The intrapulmonary pressure is subatmospheric during inspiration and greater than the atmospheric pressure during expiration.
The ideal gas law can be derived from basic principles but was originally deduced from experimental measurements of Charles law that volume occupied by a gas is proportional to temperature at a fixed pressure and from Boyles law that for a fixed temperature the product PV is a constantIn the ideal gas model the volume occupied by its atoms and molecules is a. 25 When the pressure in the lung is greater than atmospheric pressure. Thats less than atmospheric pressure but the water vapor is undeniably a gas.
Which of the following mixtures are heterogenous. Avagadros law describes the relationship of _____. Boyles law describes how gases act under pressure.
Hydrostatic Relating to the pressure that liquids exert. Normally the pressure within the pleural cavity is slightly less than the atmospheric pressure which is known as negative pressure. 27 Why do we not feel uneasy even under the enormous atmospheric pressure.
An example of this is when a. Changes of the atmospheric pressure due to weather conditions or altitude directly influence the output of a gauge pressure sensor. How does atmospheric pressure affect cooking at high altitude.
Intrapleural pressure becomes greater than intrapulmonic pressure. Lets use the following models to make sense of the problem. In a homogeneous mixture.
This subatmospheric pressure is shown as -3 mmHg. During quiet expiration for example the intrapulmonary pressure may rise to at least 3 mmHg over the atmospheric pressure. The solubility of the gas The partial pressure of the gas.
Air moves from areas of low pressure to areas of high pressure until an equilibrium is reached. Keep one sensor 1 under known pressure and another sesnor 2 under atmospheric pressure and adjust if needed. The use of an oxygen breathing mixture where the ambient pressure is greater than 1 atmosphere.
States that the tiny particles in all forms of matter are in constant motion. Of a sudden increase in surface tension. In mm Hg this is.
What Are The Properties Of Gases Physical Properties Of Gases

22 3 The Process Of Breathing Anatomy Physiology
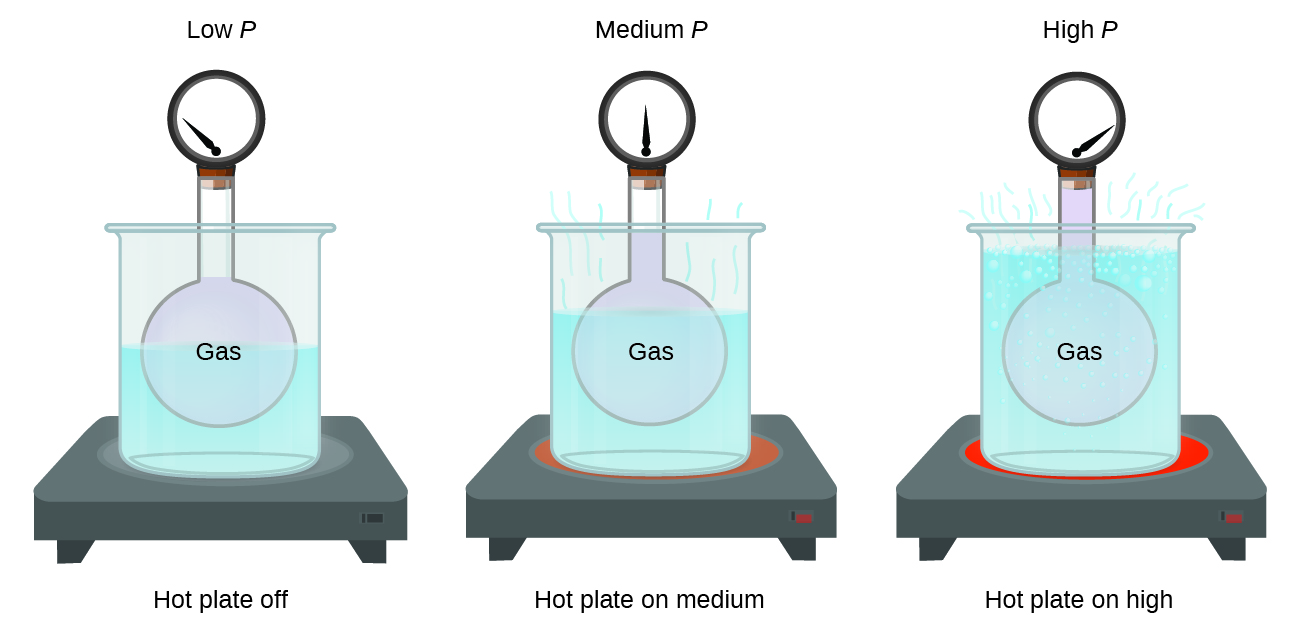
9 2 Relating Pressure Volume Amount And Temperature The Ideal Gas Law Chemistry

Manometer Pressure Problems Introduction To Barometers Measuring Gas Atmospheric Pressure Youtube

What Causes Wind Science Reading Passages Science Reading Reading Passages
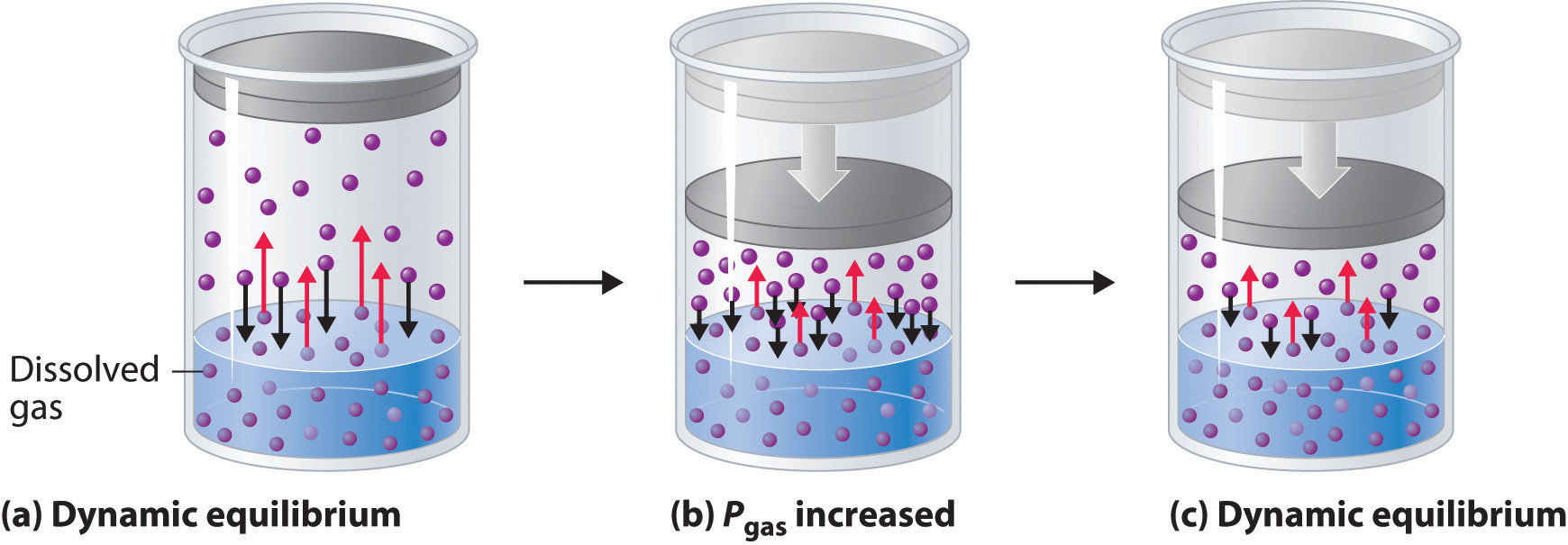
13 3 Pressure And Temperature Effects On Solubility Chemistry Libretexts

Group Definition Facts Britannica

Factors Affecting Gas Pressure Read Chemistry Ck 12 Foundation

Measuring Pressure With Barometers And Manometers Youtube
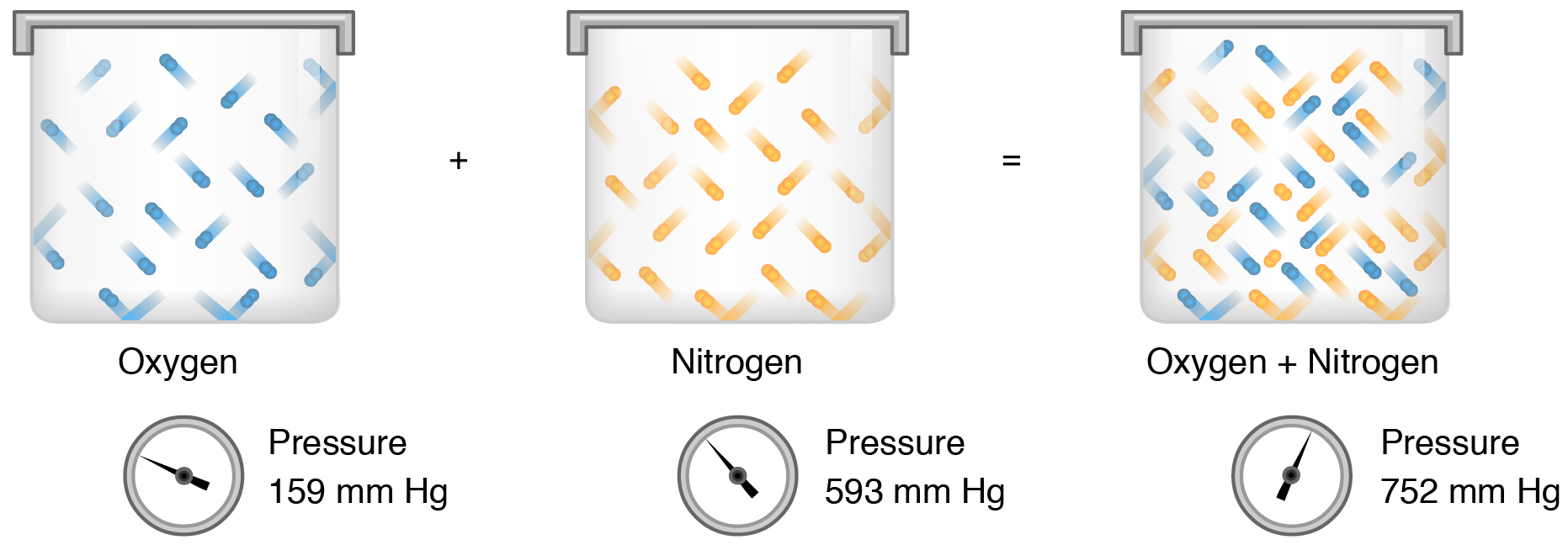
Dalton S Law Of Partial Pressure Article Khan Academy
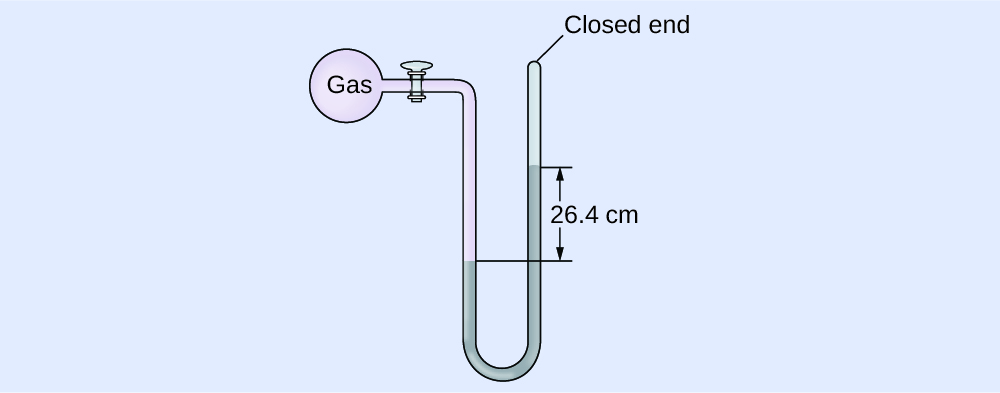
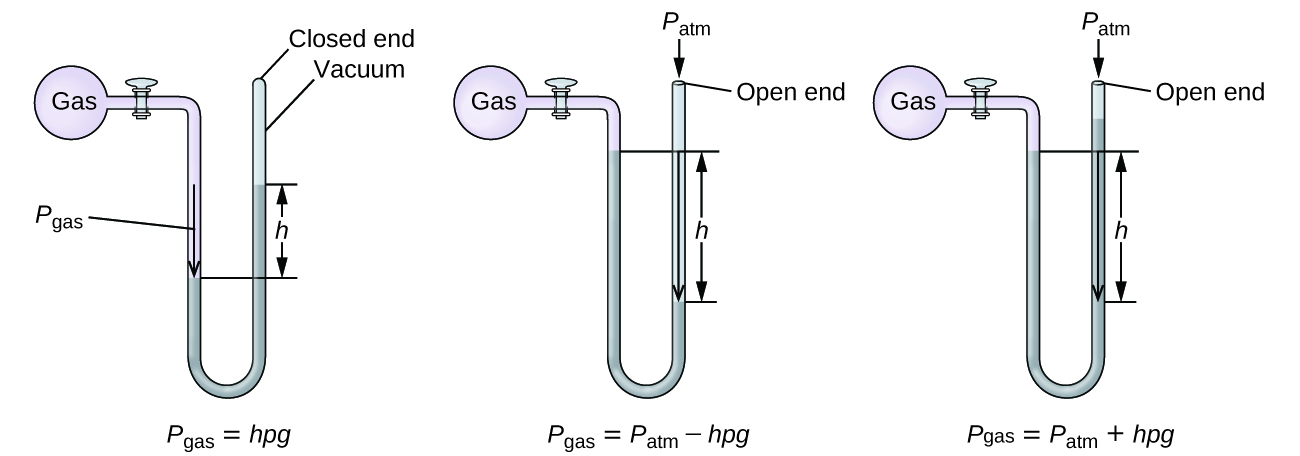
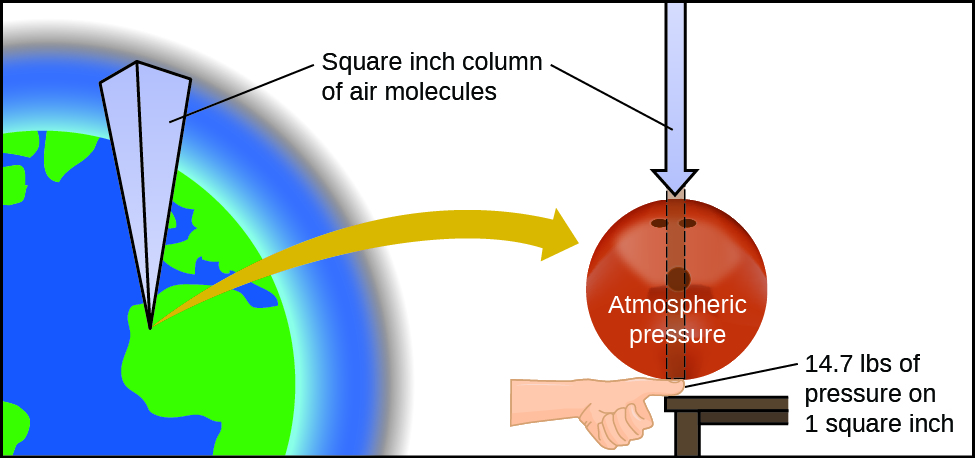


Comments
Post a Comment