Which of the Following Is Not a Strong Acid Chegg
H_2SO_4 has a pKa_1 of -3 so it dissociates very easily. Is the strongest acid.

Solved Which Of The Following Is Not A Strong Acid A Chegg Com
See the answer See the answer done loading.
. H3PO4 HNO2 H2SO3 HClO HClO2 HF H2S CH3COOH -Solutions of weak acids have a low concentration of H. Which of the following is not a strong acid in. Chlorine has highest oxidation number 7.
HNO2 Which one of the following is the weakest acid. AIEEE Bank Exams CAT. 100 of the Nitric acid will have reacted with water to produce hydronium ions and N O 3.
A H2CO3 B H2SO3 C H2SO4 D H3PO4 E CH3COOH. Chemistry questions and answers. A H 2 SO 4 B CH 3 COOH C HNO 3 D HCl Medium Solution Verified by Toppr Correct option is B Sulphuric acid nitric acid and hydrochloric acid are strong acids as in aqueous solution each of these essentially ionizes 100.
For additional organic examples see table pK a of Organic and Inorganic Acids in this appendix. A 35 is not a strong acid. Considered to be a weak acid but still dissociates its proton significantly.
HBr H2SO4 HClO4 HNO2 HI b. Tap again to see term. When the conjugate base is weak the acid is strong.
Which of the following equations describes ionization chegg com write a balanced equation for nitric acid in water tessshlo why re hydrochloric and sulfuric strong acids while hydrofluoric acetic weak oxoacids nitrogen chemistry main group elements openstax cnx showing e 16 6 libretexts what is net ionic potassium carbonate quora Which Of The Following. Which of the following is not a strong acid. It has maximum number of electronegative oxygen atoms 4 due to which more electrons will be pulled away from O-H bond and the acid will be more stronger.
The substance CH3CH22NH is considered to be A. C the equilibrium constant of each acid PAGE. This problem has been solved.
A HNO3 and H2CO3 b HI and HBr c H2SO4 and H2S d HCl and HC2H3O2 e HBr and H3PO4. Which of the following is a strong acid. A CH3NH2 B NaOH C CO2 D CaOH2 E CH4.
Acids which ionizes completely into its ions are called strong acids. Unable to ionize completely C. What is the acid that reacts with this base when ammonia is dissolved in water.
Click again to see term. This is because the conjugate base ClO 4. Has maximum delocalization due to which it is weakest base.
A highly polar molecule that contains a weak bond between a hydrogen atom and another element would be A. H_2CO_3 has a pKa_1 of about 64. Flower colours of red pink blue and purple come mainly from pigments called _______.
Which of the following is not a strong acid. A the concentration of each acid solution b the pH of each acid solution c the equilibrium constant of each acid d all of these e both a and c must be known ANS. A HCl aq B HNO3 aq C HC2H3O2 aq D HClO4 aq Expert Answer.
Ion is the weakest base. A H2CO3 b HF c H3PO4 d HCIO4 e HNO3. Which of the following is a strong acid.
5 Which of the following is not a strong acid. Which one of the following will give a solution with a pH 7 but is not an Arrhenius base in the strict sense. Which of the following is not a strong acid in aqueous solution.
We review their content and use your feedback to keep the quality high. Experts are tested by Chegg as specialists in their subject area. Which of the following is not a strong acid.
Which of the following is not a strong acid in aqueous solution. Correct option is C Among the given acids perchloric acid HClO 4. Which one of the following is a strong acid.
Which of the following is not true for a solution at 25C that has a hydroxide concentration of 25 106 M. Often called acetic acid. The hydride ion h- is a stronger base than the hydroxide ion oh- the products of the reaction of hydride ion with water are Oh- aq h2 g One of the following acids is not a strong acid.
Each of the following pairs contains one strong acid and one weak acid except. Which one of the following is a strong acid. Correct option is A OH is the strongest acid.
-The molecular form of the weak acid does exist in solution. Tap card to see definition. The weaker the conjugate base.
CH_3COOH is a carboxylic acid whose pKa is about 5 actually this one is 474. Which one of the following is a strong acid. CH3COOH chemically known as Acetic acid is not a strong acid among the given acids like Sulphuric acid Hydrochloric acid.
Which of the following is a strong acid. Ammonia acts as a weak base in aqueous solution. Analyst Bank Clerk Bank PO.
The substance NH3 is considered A a weak acid. -Any acid that is not one of the seven strong is a weak acid eg. HF pK a 35 is not a strong acid.

Solved Which Of The Following Statements Is Are True 1 At Chegg Com

Solved Which Statement Is Not True For Weak Bases B 2 O Chegg Com
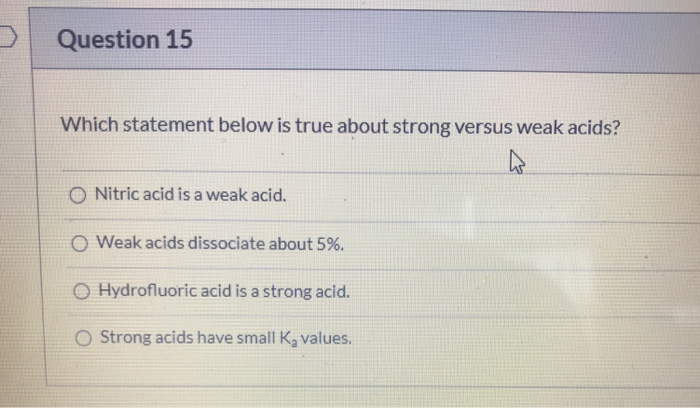
Solved Question 15 Which Statement Below Is True About Chegg Com
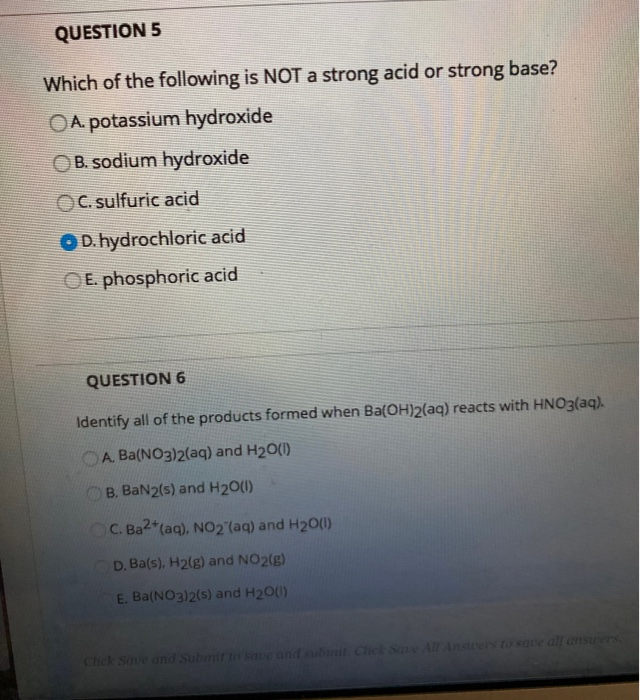
Solved Which Of The Following Is Not A Strong Acid Or Strong Chegg Com
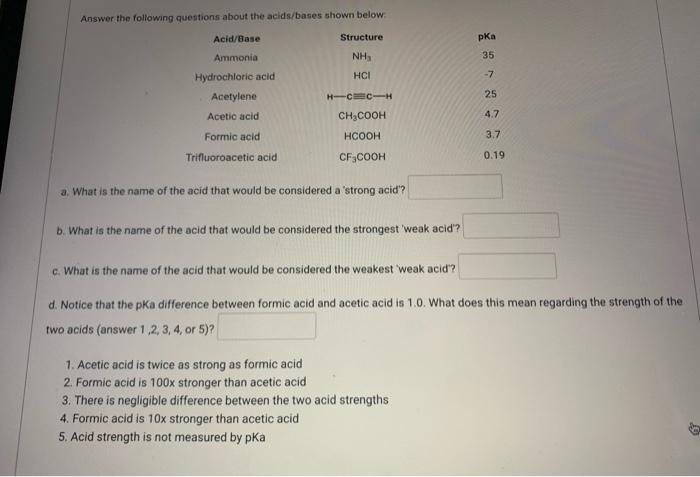
Solved Answer The Following Questions About The Acids Bases Chegg Com
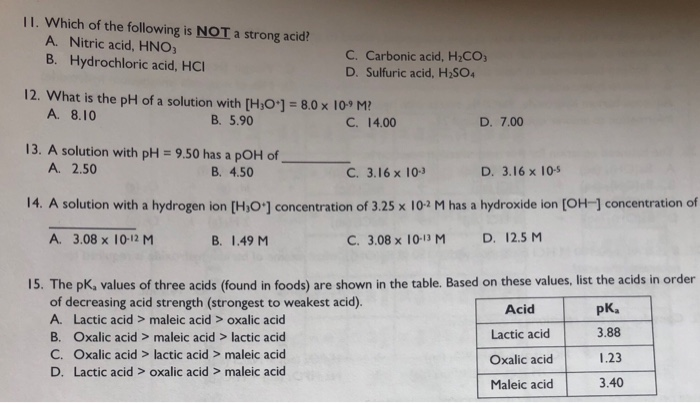
Solved Ii Which Of The Following Is Not A Strong Acid A Chegg Com

Solved Which One Of The Following Is A Strong Acid Hno2 He Chegg Com

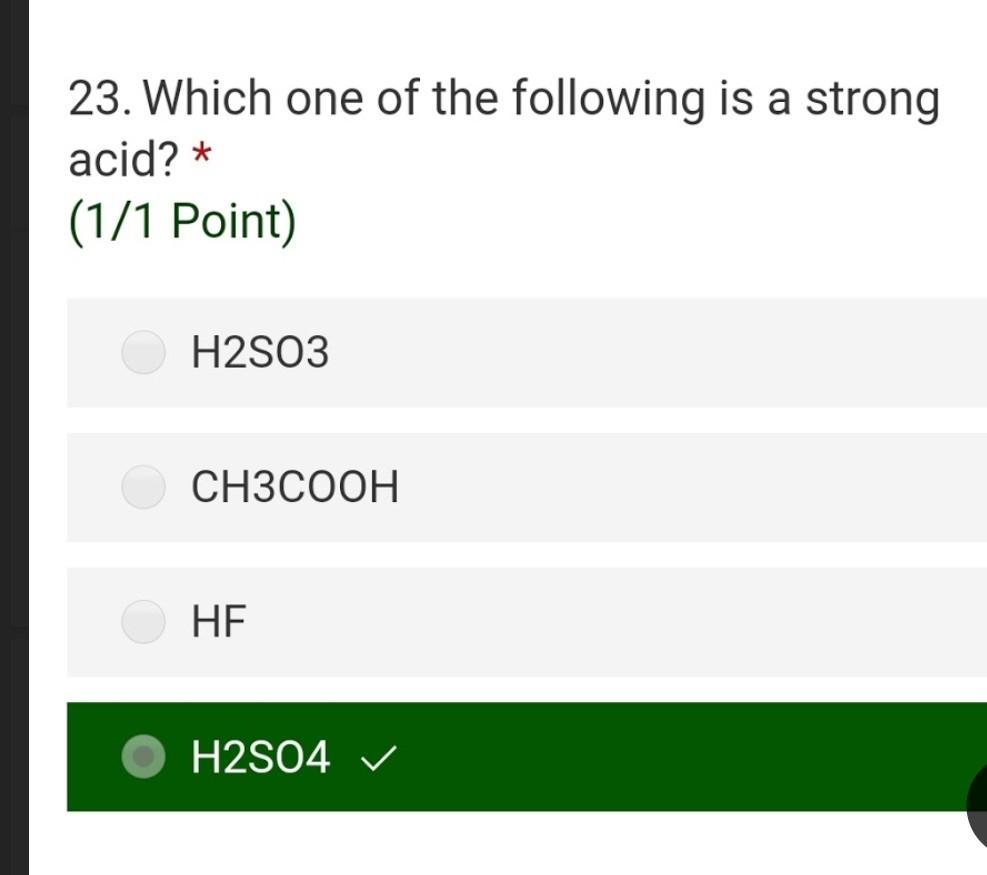
Solved 23 Which One Of The Following Is A Strong Acid Chegg Com

Solved Which Of The Following Is A Strong Acid O Nh3 Hbr Chegg Com

Solved Which Of The Following Is A Strong Acid Multiple Chegg Com

Solved 1 Which Of The Following Is Not A Strong Acid A Chegg Com

Solved 21 Which Of The Following Is A Strong Acid A Chegg Com
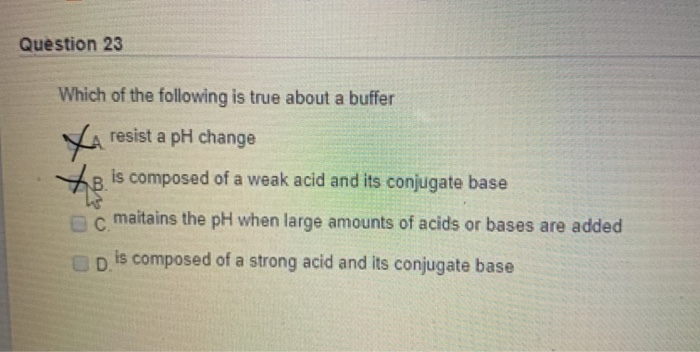
Solved Question 23 Which Of The Following Is True About A Chegg Com
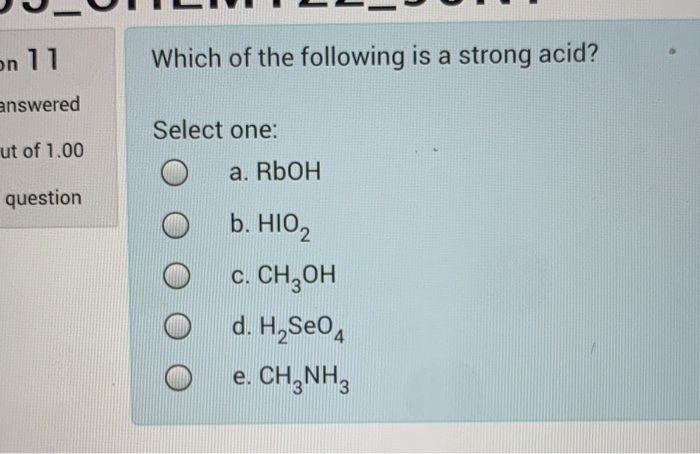
Solved On 11 Which Of The Following Is A Strong Acid Chegg Com

Solved 40 Which Of The Following Is Not A Strong Acid Hno3 Chegg Com
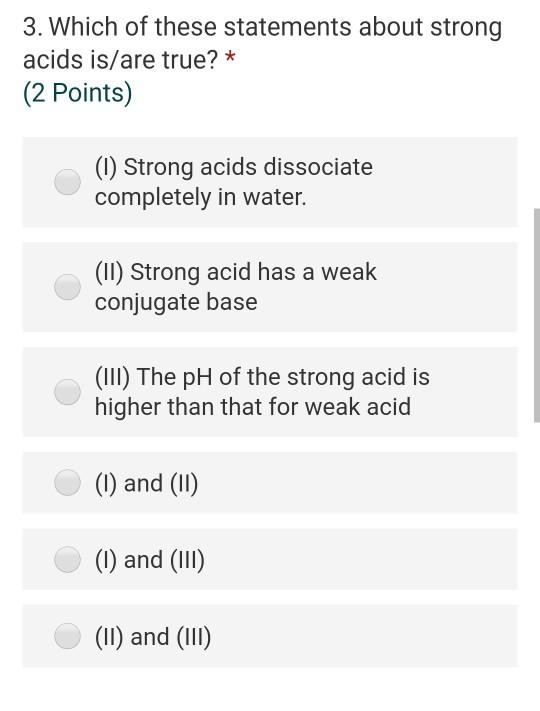
Solved 3 Which Of These Statements About Strong Acids Chegg Com
Solved Which One Of The Following Is A Strong Acid Select Chegg Com

Solved Which Of The Following Acids Is A Strong Acid A Chegg Com

Comments
Post a Comment